ORIGINAL PAPER
Effects of three-month feeding high-fat diets with different
fatty acid composition on kidney histology and expression
of genes related to cellular stress and water-electrolyte
homeostasis in mice
1
West Pomeranian University of Technology, Faculty of Biotechnology and Animal Husbandry, Department of Physiology,
Cytobiology and Proteomics, 71-270 Szczecin, Poland
2
Bydgoszcz University of Science and Technology, Faculty of Animal Breeding and Biology, Department of Animal Biotechnology
and Genetics, 85-084 Bydgoszcz, Poland
3
Pomeranian Medical University, Faculty of Health Sciences, Department of Histology and Developmental Biology,
71-210 Szczecin, Poland
4
Institute of Biochemistry and Biophysics, Polish Academy of Sciences,
Laboratory of Molecular Basis of Aging and Rejuvenation, 02-106 Warsaw, Poland
5
Institute of Genetics and Animal Biotechnology, Polish Academy of Sciences, Department of Genomics and Biodiversity,
05-552 Magdalenka, Poland
Publication date: 2023-06-22
Corresponding author
A. Wypych
West Pomeranian University of Technology, Faculty of Biotechnology and Animal Husbandry, Department of Physiology, Cytobiology and Proteomics, 71-270 Szczecin, Poland
West Pomeranian University of Technology, Faculty of Biotechnology and Animal Husbandry, Department of Physiology, Cytobiology and Proteomics, 71-270 Szczecin, Poland
J. Anim. Feed Sci. 2023;32(4):372-384
KEYWORDS
TOPICS
ABSTRACT
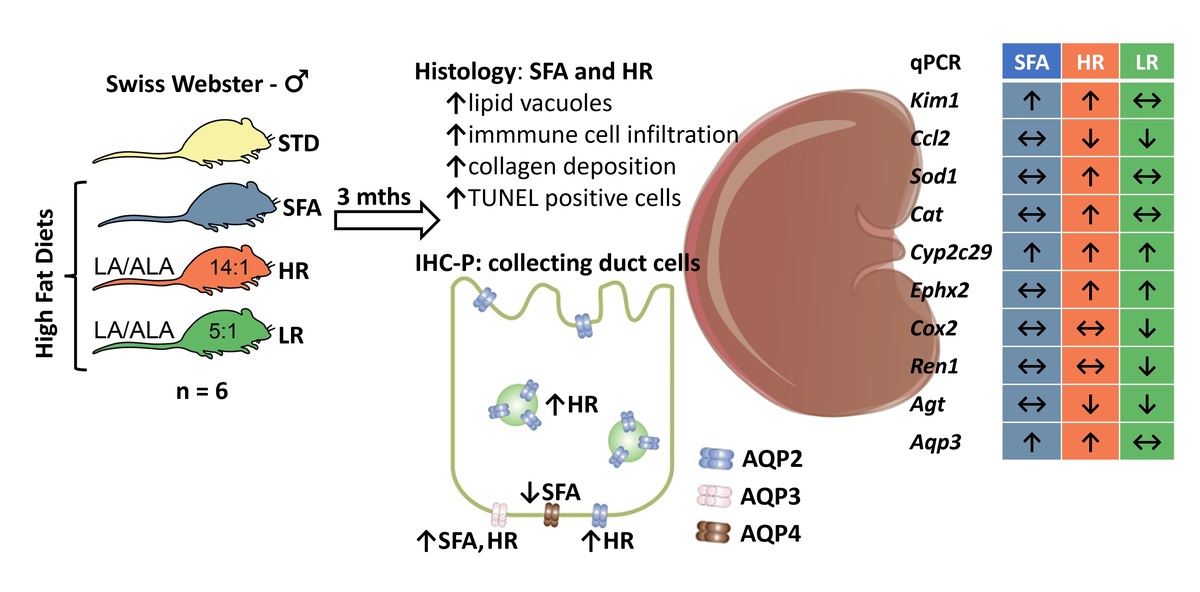
The Western diet, which is typically high in saturated fatty
acids (SFAs) and low in n-3 polyunsaturated fatty acids (PUFAs), has been
identified as a factor contributing to the growing obesity rate. Long-term
consumption of high-fat diets (HFDs) has also been associated with increased
risk of chronic kidney disease. Therefore, we hypothesized that different fatty
acids composition in HFDs would differentially affect renal microstructure and
expression pattern of selected genes. Swiss-Webster male mice (n = 24)
were fed a standard chow for mice (STD) or HFDs rich in SFAs, and rich in
PUFA with a linoleic acid (LA) to α-linolenic acid (ALA) ratio of 14:1 (HR) or
5:1 (LR) for 3 months. We observed that both the SFA and HR groups had
increased epithelial cell vacuolisation, collagenous tissue area and number
of TUNEL-positive cells, accompanied by elevated Kim-1 expression in the
kidneys. Sod1 and Cat were up-regulated, while Cox2 was down-regulated
in the kidneys of HR mice when compared to the STD group. Both PUFArich
HFDs down-regulated the Ren1 and Agt genes. The HR diet also caused
an increased deposition of AQP2 in the basolateral membrane (BLM) and
intracellular space of collecting duct (CD) cells. In both the HR and SFA groups,
an increased expression of the Aqp3 gene and AQP3 protein in CD cells was
observed. In conclusion, the findings suggest that higher levels of ALA in the
HFD were associated with a reduction in the severity of renal tissue lesions.
Diets rich in SFAs or LA have the potential to modify the renal mechanism of
facultative urine concentration by altering the expression and/or distribution of
AQP2 and AQP3 in the kidneys.
FUNDING
This work received financial support from two sources. The dietary experiment was conducted with the support of KNOW (Leading National Research Centre) Scientific Consortium “Healthy Animal – Safe Food”, decision of the Ministry of Science and Higher Education No. 05-1/KNOW2/2015”, grant no. KNOW2015/CB/PRO1/44. The analyses performed were supported by The Rector of the West Pomeranian University of Technology in Szczecin for PhD students of The Doctoral School, grant no. 35/2022.
CONFLICT OF INTEREST
The Authors declare that there is no conflict of interest.
REFERENCES (48)
1.
An W.S., Kim H.J., Cho K.H., Vaziri N.D., 2009. Omega-3 fatty acid supplementation attenuates oxidative stress, inflammation, and tubulointerstitial fibrosis in the remnant kidney. Am. J. Physiol.-Renal Physiol. 297, 895–903, https://doi.org/10.1152/ajpren....
2.
Ando F., Sohara E., Morimoto T. et al., 2016. Wnt5a induces renal AQP2 expression by activating calcineurin signalling pathway. Nat. Commun. 7, 13636, https://doi.org/10.1038/ncomms....
3.
Bordoni A., di Nunzio M., Danesi F., Biagi P.L., 2006. Polyunsaturated fatty acids: From diet to binding to ppars and other nuclear receptors. Genes Nutr. 1, 95–106, https://doi.org/10.1007/bf0282....
5.
Cheng H.F., Wang J.L., Zhang M.Z., Wang S.W., McKanna J.A., Harris R.C., 2001. Genetic deletion of COX-2 prevents increased renin expression in response to ACE inhibition. Am. J. Physiol.-Renal Physiol. 280, 449–456, https://doi.org/10.1152/ajpren....
6.
Christensen B.M., Wang W., Frøkiær J., Nielsen S., 2003. Axial heterogeneity in basolateral AQP2 localization in rat kidney: effect of vasopressin. Am. J. Physiol.-Renal Physiol. 284, 701–717, https://doi.org/10.1152/ajpren....
7.
Codde J.P., Croft K.D., Barden A., Mathews E., Vandongen R., Beilin L.J., 1984. An inhibitory effect of dietary polyunsaturated fatty acids on renin secretion in the isolated perfused rat kidney. J. Hypertens. 2, 265–270, injury and inflammatory response. Int. J. Mol. Sci. 20, 3406, https://doi.org/10.1097/000048...
8.
Costa M.C., Lima T.F.O., Arcaro C.A., Inacio M.D., Batista-Duharte A., Carlos I.Z., Spolidorio L.C., Assis R.P., Brunetti I.L., Baviera A.M., 2020. Trigonelline and curcumin alone, but not in combination, counteract oxidative stress and inflammation and increase glycation product detoxification in the liver and kidney of mice with high-fat diet-induced obesity. J. Nutr. Biochem. 76, 108303, https://doi.org/10.1016/j.jnut....
9.
Deji N., Kume S., Araki S.I. et al., 2009. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am. J. Physiol.-Renal Physiol. 296, 118–126, https://doi.org/10.1152/ajpren....
10.
Deligözoğlu D., Kasap-Demir B., Alparslan C., Erbak H., Çatlı G., Mutlubaş F., Alaygut D., Soyaltın E., Arslansoyu-Çamlar S., Yavaşcan Ö., 2020. Can we use copeptin as a biomarker for masked hypertension or metabolic syndrome in obese children and adolescents? J. Pediatr. Endocrinol. Metab. 33, 1551–1561, https://doi.org/10.1515/jpem-2....
11.
Dey A., Williams R.S., Pollock D.M., Stepp D.W., Newman J.W., Hammock B.D., Imig J.D., 2004. Altered kidney CYP2C and cyclooxygenase-2 levels are associated with obesity-related albuminuria. Obes. Res. 12, 1278–1289, https://doi.org/10.1038/oby.20....
12.
Duflot T., Laurent C., Soudey A. et al., 2021. Preservation of epoxyeicosatrienoic acid bioavailability prevents renal allograft dysfunction and cardiovascular alterations in kidney transplant recipients. Sci. Rep. 11, 3739,https://doi.org/10.1038/s41598....
13.
Duran-Montgé P., Realini C.E., Barroeta A.C., Lizardo R., Esteve-Garcia E., 2008. Tissue fatty acid composition of pigs fed different fat sources. Animal 2, 1753–1762, https://doi.org/10.1017/S17517....
14.
Ewida S.F., Al-Sharaky D.R., 2016. Implication of renal aquaporin-3 in fructose-induced metabolic syndrome and melatonin protection. J. Clin. Diag. Res. 10, CF06–CF11, https://doi.org/10.7860/JCDR/2....
15.
Gašparović A.Č., Milković L., Rodrigues C., Mlinarić M., Soveral G., 2021. Peroxiporins are induced upon oxidative stress insult and are associated with oxidative stress resistance in colon cancer cell lines. Antioxidants 10, 1856, https://doi.org/10.3390/antiox....
16.
Gujjala S., Putakala M., Nukala S., Bangeppagari M., Ramaswamy R., Desireddy S., 2016. Renoprotective effect of Caralluma fimbriata against high-fat diet-induced oxidative stress in Wistar rats. J. Food Drug Anal. 24, 586–593, https://doi.org/10.1016/j.jfda....
17.
Halperin Kuhns V., Pluznick J., 2018. Novel differences in renal gene expression in a diet induced obesity model of diabetic nephropathy. Am. J. Physiol.-Renal Physiol. 314, F517–F530, https://doi.org/10.1152/ajpren....
18.
Hintze K.J., Benninghoff A.D., Ward R.E., 2012. Formulation of the Total Western Diet (TWD) as a basal diet for rodent cancer studies. J. Agric. Food Chem. 60, 6736–6742, https://doi.org/10.1021/jf2045....
19.
Huang S., Rutkowsky J.M., Snodgrass R.G., Ono-Moore K.D., Schneider D.A., Newman J.W., Adams S.H., Hwang D.H., 2012. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res. 53,2002–2013, https://doi.org/10.1194/jlr.D0....
20.
Kasiske B.L., O’Donnell M.P., Lee H., Kim Y., Keane W F., 1991. Impact of dietary fatty acid supplementation on renal injury in obese Zucker rats. Kidney Int. 39, 1125–1134, https://doi.org/10.1038/ki.199....
21.
Kitada M., Xu J., Ogura Y., Monno I., Koya D., 2020. Manganese superoxide dismutase dysfunction and the pathogenesis of kidney disease. Front. Physiol. 11, 755, https://doi.org/10.3389/fphys.....
22.
Kume S., Uzu T., Araki S.I. et al., 2007. Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J. Am. Soc. Nephrol. 18, 2715–2723, https://doi.org/10.1681/ASN.20....
23.
Laurentius T., Raffetseder U., Fellner C., Kob R., Nourbakhsh M., Floege J., Bertsch T., Bollheimer L.C., Ostendorf T., 2019. High-fat diet-induced obesity causes an inflammatory microenvironment in the kidneys of aging Long-Evans rats. J. Inflamm. 16, 14, https://doi.org/10.1186/s12950....
24.
Lepczyński A., Ożgo M., Michałek K., Dratwa-Chałupnik A., Grabowska M., Herosimczyk A., Liput K.P., Poławska E., Kram A., Pierzchała M., 2021. Effects of three-month feeding high fat diets with different fatty acid composition on myocardial proteome in mice. Nutrients 13, 330, https://doi.org/10.3390/nu1302....
25.
Liput K.P., Lepczyński A., Ogłuszka M., Nawrocka A., Poławska E., Grzesiak A., Ślaska B., Pareek C.S., Czarnik U., Pierzchała M., 2021. Effects of dietary n-3 and n-6 polyunsaturated fatty acids in inflammation and cancerogenesis. Int. J. Mol. Sci. 22, 6965, https://doi.org/10.3390/ijms22....
26.
Luo Y., Wu M.Y., Deng B.Q. et al., 2019. Inhibition of soluble epoxide hydrolase attenuates a high-fat diet-mediated renal injury by activating PAX2 and AMPK. Proc. Natl. Acad. Sci. USA., 116, 5154–5159, https://doi.org/10.1073/pnas.1....
27.
Mobasheri A., Wray S., Marples D., 2005. Distribution of AQP2 and AQP3 water channels in human tissue microarrays. J. Mol. Histol. 36, 1–14, https://doi.org/10.1007/s10735....
28.
Morisseau C., Inceoglu B., Schmelzer K., Tsai H.J., Jinks S.L., Hegedus C.M., Hammock B.D., 2010. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J. Lipid Res. 51, 3481–3490, https://doi.org/10.1194/jlr.M0....
29.
Pischon T., Hankinson S.E., Hotamisligil G.S., Rifai N., Willett W.C., Rimm E.B., 2003. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation 108, 155–160, https://doi.org/10.1161/01.CIR....
30.
Pozzi A., Ibanez M.R., Gatica A.E., Yang S., Wei S., Mei S., Falck J.R., Capdevila J.H., 2007. Peroxisomal proliferator-activated receptor-α-dependent inhibition of endothelial cell proliferation and tumorigenesis. J. Biol. Chem. 282, 17685–17695, https://doi.org/10.1074/jbc.M7....
31.
Quadri S.S., Culver S.A., Siragy H.M., 2016. (Pro)renin Receptor (PRR) mediates high fat diet-induced hypertension via upregulation of epithelial sodium channel. Hypertension 68, 188, https://doi.org/10.1161/hyp.68....
32.
Richard D., Kefi K., Barbe U., Bausero P., Visioli F., 2008. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 57, 451–455, https://doi.org/10.1016/j.phrs....
33.
Rombaldova M., Janovska P., Kopecky J., Kuda O., 2017. Omega-3 fatty acids promote fatty acid utilization and production of pro-resolving lipid mediators in alternatively activated adipose tissue macrophages. Biochem. Biophys. Res. Commun. 490, 1080–1085, https://doi.org/10.1016/j.bbrc....
34.
Roszer T., Ricote M., 2010. PPARs in the renal regulation of systemic blood pressure. PPAR Res. 2010, 698730, https://doi.org/10.1155/2010/6....
35.
Ruggiero C., Ehrenshaft M., Cleland E., Stadler K., 2011. High-fat diet induces an initial adaptation of mitochondrial bioenergetics in the kidney despite evident oxidative stress and mitochondrial ROS production. Am. J. Physiol. Endocrinol. Metab. 300, E1047–E1058, https://doi.org/10.1152/ajpend....
36.
Sharma A., Khan M.A.H., Levick S.P., Lee K.S.S., Hammock B.D., Imig J.D., 2016. Novel omega-3 fatty acid epoxygenase metabolite reduces kidney fibrosis. Int. J. Mol. Sci. 17, 751, https://doi.org/10.3390/ijms17....
37.
Simopoulos A.P., 2008. The omega-6/omega-3 fatty acid ratio, genetic variation, and cardiovascular disease. Asia Pac. J. Clin. Nutr. 17, 131–134.
38.
Sun Y., Ge X., Li X. et al., 2020. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis. 11, 914, https://doi.org/10.1038/s41419....
39.
Szeto H.H., Liu S., Soong Y., Alam N., Prusky G.T., Seshan S.V., 2016. Protection of mitochondria prevents high-fat diet–induced glomerulopathy and proximal tubular injury. Kidney Int. 90, 997–1011, https://doi.org/10.1016/j.kint....
40.
Tang C., Cai J., Dong Z., 2016. Mitochondrial dysfunction in obesity-related kidney disease: a novel therapeutic target. Kidney Int. 90, 930–933, https://doi.org/10.1016/j.kint....
41.
Than W.H., Chan G.C.-K., Ng J. K.-C., Szeto C.C., 2020. The role of obesity on chronic kidney disease development, progression, and cardiovascular complications. Adv. Biomarker Sci. Technol. 2, 24–34, https://doi.org/10.1016/j.abst....
42.
Turolo S., Edefonti A., Mazzocchi A., Syren M.L., Morello W., Agostoni C., Montini G., 2021. Role of arachidonic acid and its metabolites in the biological and clinical manifestations of idiopathic nephrotic syndrome. Int. J. Mol. Sci. 22, 5452, https://doi.org/10.3390/ijms22....
43.
Ulu A., Harris T.R., Morisseau C. et al., 2013. Anti-inflammatory effects of ω-3 polyunsaturated fatty acids and soluble epoxide hydrolase inhibitors in angiotensin-II-dependent hypertension. J. Cardiovasc. Pharmacol. 62, 285–297, https://doi.org/10.1097/FJC.0b....
44.
Ulu A., Stephen Lee K.S., Miyabe C., Yang J., Hammock B.G., Dong H., Hammock B.D., 2014. An omega-3 epoxide of docosahexaenoic acid lowers blood pressure in angiotensin-II-dependent hypertension. J. Cardiovasc. Pharmacol. 64, 87–99, https://doi.org/10.1097/FJC.00....
45.
We R., Laboratories C.R., Zucker O., 1999. A high-fat diet aggravates tubulointerstitial but not glomerular lesions in obese Zucker rats. Kidney Int. 56, S150–S152, https://doi.org/10.1046/j.1523....
46.
Wickman C., Kramer H., 2013. Obesity and kidney disease: potential mechanisms. Semin. Nephrol. 33, 14–22, https://doi.org/10.1016/j.semn....
47.
Zeng Z., Yang H., Wang Y., Ren J., Dai Y., Dai C., 2017. Omega-3 polyunsaturated fatty acids attenuate fibroblast activation and kidney fibrosis involving MTORC2 signaling suppression. Sci. Rep. 7, 46146, https://doi.org/10.1038/srep46....
48.
Zhao X., Chen X., Zhang Y., George J., Cobbs A., Wang G., Li L., Emmett N., 2019. Kidney injury molecule-1 is upregulated in renal lipotoxicity and mediates palmitate-induced tubular cell injury and inflammatory response. Int. J. Mol. Sci. 20, 3406, https://doi.org/10.3390/ijms20....
CITATIONS (2):
1.
Kidney morphology and renal expression of aquaporins 2, 3 and 4 during cerulein – Induced chronic pancreatitis in pigs
Katarzyna Michałek, Patrycja Oberska, Maciej Murawski, Tomasz Schwarz, Ewa Tomaszewska, Siemowit Muszyński, Małgorzata Świątkiewicz, Łukasz Korytkowski, Joanna Bonior, Mateusz Zelent, David Ayomide, Marta Grabowska
Advances in Medical Sciences
Katarzyna Michałek, Patrycja Oberska, Maciej Murawski, Tomasz Schwarz, Ewa Tomaszewska, Siemowit Muszyński, Małgorzata Świątkiewicz, Łukasz Korytkowski, Joanna Bonior, Mateusz Zelent, David Ayomide, Marta Grabowska
Advances in Medical Sciences
2.
Effect of feeding high fat diets differing in fatty acid composition on oxidative stress markers and protein expression in mouse kidney
A. Wypych, M. Ożgo, M. Bernaciak, A. Herosimczyk, M. Barszcz, K. Gawin, A. K. Ciechanowicz, M. Kucia, M. Pierzchała, E. Poławska, A. Lepczyński
Journal of Animal and Feed Sciences
A. Wypych, M. Ożgo, M. Bernaciak, A. Herosimczyk, M. Barszcz, K. Gawin, A. K. Ciechanowicz, M. Kucia, M. Pierzchała, E. Poławska, A. Lepczyński
Journal of Animal and Feed Sciences
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.