ORIGINAL PAPER
Relationship between cognitive functions, levels of NR2A
and NR2B subunits of hippocampal NMDA receptors, serum
TGF-β1 level, and oxidative stress in rats fed a high-fat diet
1
Mugla Sitki Kocman University, Faculty of Medicine, Department of Physiology, 48000, Mugla, Turkey
2
Adnan Menderes University, Faculty of Medicine, Department of Physiology, 09000, Aydin, Turkey
3
Mugla Sitki Kocman University, Faculty of Science, Department of Biology, 48000, Mugla, Turkey
4
Suleyman Demirel University, Faculty of Medicine, Department of Biochemistry, 32000, Isparta, Turkey
Publication date: 2022-09-21
J. Anim. Feed Sci. 2022;31(4):318-327
KEYWORDS
TOPICS
ABSTRACT
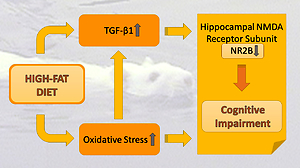
Although, excessive caloric intake is known to cause cognitive
impairment, the possible mechanism behind this phenomenon is still unknown.
Several studies have reported subunit composition changes in hippocampal
N-methyl-D-aspartate (NMDA) receptors in rats fed a high-fat diet (HFD). The
aim of this study was to test whether potential changes in hippocampal NMDA
receptor subunits, which could occur as a result of a HFD, were associated
with cognitive impairment, and to investigate their relationship with transforming
growth factor-beta 1 (TGF-β1), a cytokine associated with inflammatory events
and oxidative stress, which both have been shown to increase obesity. Two
groups of rats were formed, one fed a HFD and the other standard chow. After
feeding for 23 weeks, the rats’ cognitive functions were evaluated using the
Morris water maze test. The hippocampi of rats were homogenized and the
density of NR2A and NR2B subunits of NMDA receptors was determined. Serum
levels of TGF-β1 and malondialdehyde (MDA) were measured. While feeding
a HFD caused cognitive impairment, decreased production of the hippocampal
NR2B subunit protein, as well as increased serum TGF-β1 and MDA levels,
it did not affect the production of the hippocampal NR2A subunit. In addition,
a significant correlation was observed between impaired cognitive function and
decreased NR2B concentration and increased MDA and TGF-β1 serum levels.
Structural changes are likely to occur at the receptor level in the hippocampus
as a result of events that increase oxidative stress and TGF-β1 levels in rats
fed a HFD, thereby adversely affecting cognitive functions. TGF-β1 may be
a signalling molecule that triggers cognitive impairment.
FUNDING
This study was supported by the Adnan
Menderes University (Grant No: TPF-15016).
CONFLICT OF INTEREST
The Authors declare that there is no conflict of
interest.
METADATA IN OTHER LANGUAGES:
Chinese
高脂饮食大鼠认知功能、海马NMDA受体NR2A和NR2B亚基水
平及血清TGF -β1水平与氧化应激的关系
摘要: 虽然已知能量摄入过量会导致认知障碍,但这种现象背后的可能机制仍不清楚。有研究报道认为饲
喂高脂饮食(high-fat diet,HFD)大鼠的海马NMDA受体的亚单位组成发生变化。本研究的目的是测定海马区
NMDA受体亚单位潜在变化是否与认知障碍有关,并探讨它们与转化生长因子β1(transforming growth factor-beta
1,TGF-β1)的关系,转化生长因子β1是一种与炎症和氧化应激相关的细胞因子,两者都已被证明会导致肥
胖。 试验分为两组,一组饲喂HFD,另一组饲喂标准饲料。饲养23周后,通过水迷宫试验测试大鼠的认知
功能。取大鼠海马匀浆,测定NMDA受体NR2A和NR2B亚基的水平。检测血清TGF-β1和丙二醛(malondialdehyde,MDA)的水平。饲喂HFD可引起大鼠认知功能障碍,降低海马NR2B亚单位蛋白的生成,增加血清TGF-β1
和MDA水平,但不影响海马NR2A亚单位的生成。此外,认知功能损害与NR2B浓度降低,血清TGF-β1和MDA
水平升高显著相关。饲喂HFD的大鼠,由于氧化应激和TGF-β1水平增加,可能导致海马区受体水平发生结构
变化,从而对认知功能产生不利影响。TGF-β1可能是一种触发认知功能障碍的信号因子。
REFERENCES (41)
1.
Agustí A., García-Pardo M.P., López-Almela I., Campillo I., Maes M., Romaní-Pérez M., Sanz Y., 2018. Interplay between the gutbrain axis, obesity and cognitive function. Front. Neurosci. 12, 155, https://doi.org/10.3389/fnins....
2.
Ayala A., Muñoz M.F., Argüelles, S., 2014. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 1–31, https://doi.org/10.1155/2014/3...
3.
Bagaitkar J., Pech N.K., Ivanov S., Austin A., Zeng M.Y., Pallat S., Huang G., Randolph G.J., Dinauer M.C., 2015. NADPH oxidase controls neutrophilic response to sterile inflammation in mice by regulating the IL-1α/G-CSF axis. Blood 126, 2724–2733, https://doi.org/10.1182/blood-...
4.
Barretto J.R., Boa-Sorte N., Vinhaes C.L., Malta-Santos H., Rebouças-Silva J., Ramos C.F., Torres-Nascimento M.A.S., Borges V.M., Andrade B.B., 2020. Heightened plasma levels of transforming growth factor beta (TGF-β) and increased degree of systemic biochemical perturbation characterizes hepatic steatosis in overweight pediatric patients: a crosssectional study. Nutrients 12, 1650, https://doi.org/10.3390/nu1206...
5.
Buie J.J., Watson L.S., Smith C.J., Sims-Robinson C., 2019. Obesityrelated cognitive impairment: The role of endothelial dysfunction. Neurobiol. Dis. 132, 104580, https://doi.org/10.1016/j.nbd....
6.
Caraci F., Gulisano W., Guida C.A., Impellizzeri A.A.R., Drago F., Puzzo D., Palmeri A., 2015. A key role for TGF-β1 in hippocampal synaptic plasticity and memory. Sci. Rep. 5, 11252, https://doi.org/10.1038/srep11...
7.
Davis J.A., Paul J.R., McMeekin L.J., Nason S.R., Antipenko J.P., Yates S.D., Cowell R.M., Habegger K.M., Gamble, K.L., 2020. High-fat and high-sucrose diets impair time-of-day differences in spatial working memory of male mice. Obesity 28, 2347–2356, https://doi.org/10.1002/oby.22...
8.
Ding L., Kang Y., Dai H.-B. et al., 2019. Adipose afferent reflex is enhanced by TNF-α in paraventricular nucleus through NADPH oxidase-dependent ROS generation in obesity-related hypertensive rats. J. Transl. Med. 17, 256, https://doi.org/10.1186/s12967...
9.
Duarte J., 2014. Metabolic alterations associated to brain dysfunction in diabetes. Aging Dis. 6, https://doi.org/10.14336/ad.20...
10.
Eyre H., Kahn R., Robertson R.M. et al., 2004. Preventing cancer, cardiovascular disease, and diabetes. Circulation 109, 3244–3255, https://doi.org/10.1161/01.CIR...
11.
Farhangi M.A., Nameni G., Hajiluian G., Mesgari-Abbasi M., 2017. Cardiac tissue oxidative stress and inflammation after vitamin D administrations in high fat-diet induced obese rats. BMC Cardiovasc. Disord. 17, 161, https://doi.org/10.1186/s12872...
12.
Franchini L., Carrano N., di Luca M., Gardoni F., 2020. Synaptic GluN2A-containing NMDA receptors: from physiology to pathological synaptic plasticity. Int. J. Mol. Sci. 21, 1538, https://doi.org/10.3390/ijms21...
13.
Gyengesi E., Paxinos G., Andrews Z.B., 2012. Oxidative stress in the hypothalamus: the importance of calcium signaling and mitochondrial ROS in body weight regulation. Curr. Neuropharmacol. 10, 344–353, https://doi.org/10.2174/157015...
14.
Haddad-Tóvolli R., Dragano N.R.V., Ramalho A.F.S., Velloso L.A., 2017. Development and function of the blood-brain barrier in the context of metabolic control. Front. Neurosci. 11, https://doi.org/10.3389/fnins....
15.
Hong S.-H., Kang M., Lee K.-S., Yu K., 2016. High fat diet-induced TGF-β/Gbb signaling provokes insulin resistance through the tribbles expression. Sci. Rep. 6, 30265, https://doi.org/10.1038/srep30...
16.
Joffe Y.T., Collins M., Goedecke J.H., 2013. The relationship between dietary fatty acids and inflammatory genes on the obese phenotype and serum lipids. Nutrients 5, 1672–1705, https://doi.org/10.3390/nu5051...
17.
Jung Y.-H., Suh Y.-H., 2010. Differential functions of NR2A and NR2B in short-term and long-term memory in rats. NeuroReport 21, 808-811, https://doi.org/10.1097/WNR.0b...
18.
Kashima R., Hata A., 2018. The role of TGF-β superfamily signaling in neurological disorders. Acta Biochim. Biophys. Sin. 50, 106–120, https://doi.org/10.1093/abbs/g...
19.
Korn T., Bettelli E., Oukka M., Kuchroo V.K., 2009. IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485-517, https://doi.org/10.1146/annure...
20.
Laemmli U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685, https://doi.org/10.1038/227680...
21.
Lee M.-J., 2018. Transforming growth factor beta superfamily regulation of adipose tissue biology in obesity. Biochim. Biophys. Acta (BBA) - Molecular Basis of Disease 1864, 1160–1171, https://doi.org/10.1016/j.bbad...
22.
Lin E., Kuo P.-H., Liu Y.-L., Yang A.C., Tsai, S.-J., 2017. Transforming growth factor-β signaling pathway-associated genes SMAD2 and TGFBR2 are implicated in metabolic syndrome in a Taiwanese population. Sci. Rep. 7, 13589, https://doi.org/10.1038/s41598...
23.
Liu Y., Yu J., Shi Y.-C., Zhang Y., Lin S., 2019. The role of inflammation and endoplasmic reticulum stress in obesity-related cognitive impairment. Life Sci. 233, 116707, https://doi.org/10.1016/j.lfs....
24.
Luciano-Jaramillo J., Sandoval-García F., Vázquez-Del Mercado M. et al., 2019. Downregulation of hippocampal NR2A/2B subunits related to cognitive impairment in a pristane-induced lupus BALB/c mice. PLoS ONE 14, e0217190, https://doi.org/10.1371/journa...
25.
Melo H.M., Seixas da Silva G. da S., Sant’Ana M.R. et al., 2020. Palmitate is increased in the cerebrospinal fluid of humans with obesity and induces memory impairment in mice via proinflammatory TNF-α. Cell Rep. 30, 2180–2194.e8, https://doi.org/10.1016/j.celr...
26.
Meo S.A., Altuwaym A.A., Alfallaj R.M., Alduraibi K.A., Alhamoudi A.M., Alghamdi S.M., Akram A., 2019. Effect of obesity on cognitive function among school adolescents: a cross-sectional study. Obes. Facts 12, 150–156, https://doi.org/10.1159/000499...
27.
Miller A.A., Spencer S.J., 2014. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav. Immun. 42, 10–21, https://doi.org/10.1016/j.bbi....
28.
Moheet A., Mangia S., Seaquist E.R., 2015. Impact of diabetes on cognitive function and brain structure. Ann. NY Acad. Sci. 1353, 60–71, https://doi.org/10.1111/nyas.1...
29.
Prem P.N., Kurian G.A., 2021. High-fat diet increased oxidative stress and mitochondrial dysfunction induced by renal ischemiareperfusion injury in rat. Front. Physiol. 12, 1353, https://doi.org/10.3389/fphys....
30.
Prud’homme G.J., 2007. Pathobiology of transforming growth factor β in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab. Invest. 87, 1077–1091, https://doi.org/10.1038/labinv...
31.
Rom S., Zuluaga-Ramirez V., Gajghate S., Seliga A., Winfield M., Heldt N.A., Kolpakov M.A., Bashkirova Y.V., Sabri A.K., Persidsky Y., 2019. Hyperglycemia-driven neuroinflammation compromises BBB leading to memory loss in both diabetes mellitus (DM) type 1 and type 2 mouse models. Mol. Neurobiol. 56, 1883–1896, https://doi.org/10.1007/s12035...
32.
Romieu I., Dossus L., Barquera S. et al., 2017. Energy balance and obesity: what are the main drivers? Cancer Causes Control 28, 247–258. https://doi.org/10.1007/s10552...
33.
Sanjabi S., Zenewicz L.A., Kamanaka M., Flavell, R.A., 2009. Anti-inflammatory and pro-inflammatory roles of TGF-β, IL-10, and IL-22 in immunity and autoimmunity. Curr. Opin. Pharmacol. 9, 447-453, https://doi.org/10.1016/j.coph...
34.
Seshiah P.N., Weber D.S., Rocic P., Valppu L., Taniyama Y., Griendling K.K., 2002. Angiotensin II stimulation of NAD(P) H oxidase activity. Circ. Res. 91, 406–413, https://doi.org/10.1161/01.RES...
35.
Silva Junior G.B. da, Bentes A.C.S.N., Daher E.D.F., Matos S.M.A. de., 2017. Obesity and kidney disease. J. Bras. Nefrol. 39, https://doi.org/10.5935/0101-2...
36.
Tan B.L., Norhaizan M.E., 2019a. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients 11, 2579, https://doi.org/10.3390/nu1111...
37.
Tan B.L., Norhaizan M.E., Liew W.P.P., Rahman H.S., 2018. Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front. Pharmacol. 9, 1162, https://doi.org/10.3389/fphar....
38.
Trevisan M., Browne R., Ram M., Muti P., Freudenheim J., Carosella A.M., Armstrong D., 2001. Correlates of markers of oxidative status in the general population. Am. J. Epidemiol. 154, 348–356, https://doi.org/10.1093/aje/15...
39.
WHO (World Health Organization), 2021. World Health Organization fact sheets: obesity and overweight (updated 9 June 2021), https://www.who.int/news-room/...
40.
Yilmaz N., Vural H., Yilmaz M., Sutcu R., Sirmali R., Hicyilmaz H., Delibas N., 2011. Calorie restriction modulates hippocampal NMDA receptors in diet-induced obese rats. J. Recept. Signal Transduct. 31, https://doi.org/10.3109/107998...
41.
Yuzefovych L.V., Musiyenko S.I., Wilson G.L., Rachek L.I., 2013. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat dietinduced insulin resistance mice. PLoS ONE 8, e54059, https://doi.org/10.1371/journa...
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.