Journal IF and Current issue
Online first
Archive
About the Journal
Editorial Office
Editorial Board
Copy right and self-archiving policy
Peer review process
Instructions for Reviewers
Printed version subscription
Abstracting and indexing
Contact
Instructions for Authors
Policies
General information
Open Access, Licensing terms, Commercial use and Copyright terms policies
Self-archiving policy and Archive policies
Article Correction and Withdrawal policy
Manuscript Submission policy
Authorship policy
Conflict of Interest policy
Language considerations policy
Plagiarism and Duplicate publications policy
Ethics policy
Review process policy
Acceptance of manuscripts policy
Online First Articles and Special Issues policies
Generative artificial intelligence (AI) policy
Advertising policy
Article publication charges
Policies
General information
Open Access, Licensing terms, Commercial use and Copyright terms policies
Self-archiving policy and Archive policies
Article Correction and Withdrawal policy
Manuscript Submission policy
Authorship policy
Conflict of Interest policy
Language considerations policy
Plagiarism and Duplicate publications policy
Ethics policy
Review process policy
Acceptance of manuscripts policy
Online First Articles and Special Issues policies
Generative artificial intelligence (AI) policy
Advertising policy
ORIGINAL PAPER
Assessment of the ability of dietary yeast-fermented rapeseed
meal to modulate inflammatory and oxidative stress
in piglets after weaning
1
National Research-Development Institute for Animal Biology and Nutrition (INCDBNA-IBNA), Balotesti, 077015 Ilfov, Romania
2
Babeş Bolyai University, Raluca Ripan Institute for Research in Chemistry,
400294 Cluj-Napoca, Romania
Publication date: 2022-05-24
J. Anim. Feed Sci. 2022;31(2):109-122
KEYWORDS
TOPICS
ABSTRACT
The aim of the study was to investigate the potential of a diet containing
rapeseed meal fermented by Saccharomyces cerevisiae as a new sustainable
feed to reduce transient intestinal inflammation, diarrhoea and oxidative
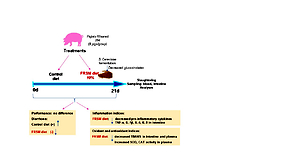
stress in piglets after weaning. In this study, 16 male post-weaning piglets, with an
initial weight of 9.04 ± 0.19 kg, were randomly allocated to two dietary treatments:
control and 10% fermented rapeseed meal (FRSM) – 8 pigs/treatment. The experiment
lasted 21 days. At the end of the trial, the animals were slaughtered and
samples of blood and segments of the jejunum, ileum and colon were collected
for determination of plasma biochemical, inflammatory and oxidative stress parameters.
Pig performance and diarrhoea incidence were also investigated. The
results showed that the FRSM diet had no significant effect on piglet performance,
weight and average daily weight gain, as well as plasma biochemical parameters.
However, the number of piglets with diarrhoea was higher in the control group
than in the group receiving the FRSM diet throughout the experimental period.
Moreover, a decrease in TNF-α (P = 0.03) and IL-1ß (P < 0.05) cytokine levels
was recorded in the colon and jejunum samples from the FRSM group. In addition,
IL-8 and IL-6 concentrations were decreased (P = 0.0009 and P = 0.03,
respectively) in the ileum of piglets fed FRSM, indicating the modulatory capacity
of this feed in reducing weaning-associated intestinal inflammation. The FRSM
diet also improved the antioxidant status and significantly reduced lipid peroxidation
and thiobarbituric acid reactive substance levels in plasma (P = 0.022) and
in the jejunum (P = 0.028) and colon (P = 0.003), suggesting the potential of
fermented rapeseed meal to limit oxidative reactions. In conclusion, the present
study showed that fermentation of rapeseed meal using S. cerevisiae enriched the
nutrient composition and reduced the concentration of anti-nutrients (e.g. glucosinolates).
Moreover, the addition of FRSM to diets of pigs after weaning improved
their intestinal health status, indicating its beneficial effect.
FUNDING
This research was funded by the Romanian
Ministry of Education and Research under the project
No. PN-III-P2-2.1-PED/396-2020.
CONFLICT OF INTEREST
The Authors declare that there is no conflict of
interest.
METADATA IN OTHER LANGUAGES:
Chinese
酵母发酵菜籽粕对断奶仔猪炎症和氧化应激调控能力的评价
摘要:本研究旨在探讨含酵母菌发酵的菜籽粕作为一种新型可持续饲料降低仔猪断奶后短暂肠道炎症、腹
泻和氧化应激的潜力。试验选取16头初始体重为9.04 ± 0.19 kg的断奶后公仔猪,随机分为对照组和10%发
酵菜籽粕(FRSM)组(8头/组),试验期为21 d。试验结束后,屠宰动物,采集血液及空肠、回肠和结肠
部分样本,测定血浆生化、炎症和氧化应激参数。并对猪的生产性能和腹泻发生率进行了调查。结果表
明:FRSM饲喂对仔猪生产性能、体重、平均日增重及血浆生化指标均无显著影响。试验期,对照组仔猪
腹泻数均高于FRSM组。此外,FRSM组结肠和空肠标本中TNF-α(P = 0.03)和IL-1ß(P < 0.05)细胞因子水平
下降。FRSM降低了仔猪回肠IL-8和IL-6浓度(P = 0.0009和P = 0.03),表明FRSM对降低断奶相关性肠道炎症
具有调节作用。FRSM饲喂还改善了抗氧化状态,显著降低了血浆(P = 0.022)、空肠(P = 0.028)和结肠
(P = 0.003)脂质过氧化和硫代巴比妥酸反应物质(TBARS)水平,表明发酵菜籽粕具有抑制氧化反应的潜
力。综上所述,FRSM可丰富其营养成分,降低抗营养物质(如硫代葡萄糖苷)的浓度。此外,仔猪断奶后
饲粮中添加FRSM改善了肠道健康状况,表明其对肠道具有有益的作用。
REFERENCES (74)
1.
Batumalaie K., Safi S.Z., Yusof K.M., Ismail I.S., Sekaran S.D., Qvist R., 2013. Effect of gelam honey on the oxidative stress-induced signaling pathways in pancreatic hamster cells. Int. J. Endocrinol. 2013, 367312, https://doi.org/10.1155/2013/3...
2.
Bell J.M., 1993. Factors affecting the nutritional value of canola meal: a review. Can. J. Anim. Sci. 73, 689–697, https://doi.org/10.4141/cjas93...
3.
Campbell J.M., Crenshaw J.D., Polo J., 2013. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 4, https://doi.org/10.1186/2049-1...
4.
Chen C., de Nanclares M.P., Kurtz J.F., et al., 2018. Identification of redox imbalance as a prominent metabolic response elicited by rapeseed feeding in swine metabolome. J. Anim. Sci. 96, 1757–1768, https://doi.org/10.1093/jas/sk...
5.
Chiang G., Lu W.Q., Piao X.S., Piao, Hu J.K., Gong L.M., Thacker P.A., 2010. Effects of feeding solid-state fermented rapeseed meal on performance, nutrient digestibility, intestinal ecology and intestinal morphology of broiler chickens. Asian-Australas. J. Anim. Sci. 23, 263–271, https://doi.org/10.5713/ajas.2...
6.
Chuang W.Y., Lin L.J., Hsieh Y.C., Chang S.C., Lee T.T., 2021. Effects of Saccharomyces cerevisiae and phytase co-fermentation of wheat bran on growth, antioxidation, immunity and intestinal morphology in broilers. Anim. Biosci. 34, 1157–1168, https://doi.org/10.5713/ajas.2...
7.
Czech A., Sembratowicz I., Kiesz M., 2021. The effects of a fermented rapeseed or/and soybean meal additive on antioxidant parameters in the blood and tissues of piglets. Animals 11, 1646, https://doi.org/10.3390/ani110...
8.
de Groot N., Fariñas F., Cabrera-Gómez C.G., Pallares F.J., Ramis G., 2021. Weaning causes a prolonged but transient change in immune gene expression in the intestine of piglets. J. Anim. Sci. 99, https://doi.org/10.1093/jas/sk...
9.
Do S.H. Kim B.O., Fang L.H., You D.H., su Hong J., Kim Y.K., 2017. Various levels of rapeseed meal in weaning pig diets from weaning to finishing periods. Asian-Australas. J. Anim. Sci. 30, 1292–1302, https://doi.org/10.5713/ajas.1...
10.
Dragomir C., Rinne M., Ruiz D.R.Y., 2015. Novel coproducts from corn milling and their use in ruminants’ nutrition. Turk. J. Vet. Anim. 39, 245–253, https://doi.org/10.3906/vet-14...
11.
Drażbo A.A., Juśkiewicz J., Józefiak A., Konieczka P., 2020. The fermentation process improves the nutritional value of rapeseed cake for turkeys-effects on performance, gut bacterial population and its fermentative activity. Animals 10, 1711, https://doi.org/10.3390/ani100...
12.
Drazbo A, Ognik K , Zaworska A, Ferenc K, Jankowski J., 2018. The effect of raw and fermented rapeseed cake on the metabolic parameters, immune status, and intestinal morphology of turkeys. Poult Sci. 97, 3910–3920,https://doi.org/10.3382/ps/pey...
14.
Fazhi X., Lvmu L., Jiaping X., Kun Q., Zhide Z., Zhangyi L., 2011. Effects of fermented rapeseed meal on growth performance and serum parameters in ducks. Asian-Australas. J. Anim. Sci. 24, 678–684, https://doi.org/10.5713/ajas.2...
15.
Feng H., Qu H., Liu Y., Shi Y., Wu S., Bao W., 2020. Effect of fermented soybean meal supplementation on some growth performance, blood chemical parameters, and fecal microflora of finishing pigs. R. Bras. Zootec. 49, https://doi.org/10.37496/rbz49...
16.
Fukuda M. Kanauchi O., Araki Y., et al., 2002. Prebiotic treatment of experimental colitis with germinated barley foodstuff: a comparison with probiotic or antibiotic treatment. Int. J. Mol. Med. 9, 65–70, https://doi.org/10.3892/ijmm.9...
17.
Gilmore T.D., 2006. Introduction to NF-κB: players, pathways, perspectives. Oncogene 25, 6680-6684, https://doi.org/10.1038/sj.onc...
18.
Govers M.J., Gannon N.J., Dunshea F.R., Gibson P.R., Muir J.G., 1999. Wheat bran affects the site of fermentation of resistant starch and luminal indexes related to colon cancer risk: a study in pigs. Gut 45, 840–847, https://doi.org/10.1136/gut.45...
19.
Grela E.R., Czech A., Kiesz M., Wlazło Ł., Nowakowicz-Dębek B., 2019. A fermented rapeseed meal additive: effects on production performance, nutrient digestibility, colostrum immunoglobulin content and microbial flora in sows. Anim. Nutr. 5, 373–379, https://doi.org/10.1016/j.anin...
20.
Hong K.J., Lee C.-H., Kim S.W., 2004. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J. Med. Food 7, 430–435, https://doi.org/10.1089/jmf.20...
21.
Hu Y., Wang Y., Li A., Wang Z., Zhang X., Yun T., Qiu L., Yin Y., 2016. Effects of fermented rapeseed meal on antioxidant functions, serum biochemical parameters and intestinal morphology in broilers. Food Agric. Immunol. 27, 182–193, https://doi.org/10.1080/095401...
22.
ISO (International Organization for Standardization), 2010. Standardized Bulletin, SR ISO 6496/2001, http://www.asro.ro
23.
Khor T.O., Huang M.-T., Kwon K.H., Chan J.Y., Reddy B.S., Kong A.-N., 2006. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 66, 11580–11584, https://doi.org/10.1158/0008-5...
24.
La Marca M., Beffy P., Pugliese A., Longo V., 2013. Fermented wheat powder induces the antioxidant and detoxifying system in primary rat hepatocytes. PLOS ONE 8, e83538, https://doi.org/10.1371/journa...
25.
Lallès J.-P., Boudry G., Favier C., Le Floc’h N., Luron I., Montagne L., Oswald I.P., Pie C., Seve B., 2004. Gut function and dysfunction in young pigs: physiology. Anim. Res. 53, 301–316, https://doi.org/10.1051/animre...
26.
Landero J.L., Beltranena E., Cervantes M., Araiza A.B., Zijlstra R.T., 2012. The effect of feeding expeller-pressed canola meal on growth performance and diet nutrient digestibility in weaned pigs. Animal Feed Sci. Technol. 171, 240–245, https://doi.org/10.1016/j.anif...
27.
Landero J.L., Beltranena E., Cervantes M., Morales A., Zijlstra R.T., 2011. The effect of feeding solvent-extracted canola meal on growth performance and diet nutrient digestibility in weaned pigs. Anim. Feed Sci. Technol. 170, 136–140, https://doi.org/10.1016/j.anif...
28.
Landero J.L., Wang L.F., Beltranena E., Bench C.J., Zijlstra R.T., 2018. Feed preference of weaned pigs fed diets containing soybean meal, Brassica napus canola meal, or Brassica juncea canola meal. J. Anim. Sci. 96, 600–611, https://doi.org/10.1093/jas/sk...
29.
Lee J.O., Park M.H., Choi Y.H., Ha Y.L., Ryu C.H., 2007. New fermentation technique for complete digestion of soybean protein. J. Microbiol. Biotechnol. 17, 1904–1907
30.
Li P., Lyu Z., Wang L., Huang B., Lai C., 2020. Nutritive values of double-low rapeseed expellers and rapeseed meal with or without supplementation of multi-enzyme in pigs. Can. J. Anim. Sci. 100, 729–738, https://doi.org/10.1139/cjas-2...
31.
Lin W., Wu R.T., Wu T., Khor T.-O., Wang H., Kong A.-N., 2008. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochemical pharmacology 76, 967–973, https://doi.org/10.1016/j.bcp....
32.
Long S., Liu L., Liu S., Mahfuz S., Piao X., 2019. Effects of Forsythia Suspense extract as an antibiotics substitute on growth performance, nutrient digestibility, serum antioxidant capacity, fecal Escherichia coli concentration and intestinal morphology of weaned piglets. Animals 9, 729, https://doi.org/10.3390/ani910...
33.
Long S., Ma J., Piao X., Li Y., Rasmussen S.H., Liu L., 2021. Enzymetreated soybean meal enhanced performance via improving immune response, intestinal morphology and barrier function of nursery pigs in antibiotic free diets. Animals 11, 2600, https://doi.org/10.3390/ani110...
34.
Maribo H., Saur C., 2012. Fermented rapeseed for weaners. Pig Research Centre. Trial report no. 942
35.
Marin D., Bulgaru C.V., Anghel C.A., Pistol G.C., Dore M.I., Palade M.L., Taranu I., 2020. grape seed waste counteracts aflatoxin B1 toxicity in piglet mesenteric lymph nodes. Toxins 12, 800, https://doi.org/10.3390/toxins...
36.
Marin D.E., Pistol G.C., Neagoe I.V., Calin L., Taranu I., 2013. Effects of zearalenone on oxidative stress and inflammation in weanling piglets. Food Chem. Toxicol. 58, 408–415, https://doi.org/10.1016/j.fct....
37.
Mejicanos G., Sanjayan N., Kim I.H., Nyachoti C.M., 2016. Recent advances in canola meal utilization in swine nutrition. J. Anim Sci. Technol. 58, 7, https://doi.org/10.1186/s40781...
38.
Mendivil E.J., Sandoval-Rodriguez A., Meza-Rios A., Zuniga-Ramos L., Dominguez-Rosales A, Mercado M.V., Sanchez-Orozco L., Santos-Garcia A., Armendariz-Boruda J., 2019. Capsaicin induces a protective effect on gastric mucosa along with decreased expression of inflammatory molecules in a gastritis model. J. Funct. Foods 59, 345–351, https://doi.org/10.1016/j.jff....
39.
Mizumachi K., Aoki R., Ohmori H., Saeki M., Kawashima T., 2009. Effect of fermented liquid diet prepared with Lactobacillus plantarum LQ80 on the immune response in weaning pigs. Animal 3, 670–676, https://doi.org/10.1017/S17517...
40.
Nakanishi Y., Murashima K., Ohara H. et al., 2006. Increase in terminal restriction fragments of Bacteroidetes-derived 16S rRNA genes after administration of short-chain fructooligosaccharides. Appl. Environ. Microbiol. 72, 6271–6276, https://doi.org/10.1128/AEM.00...
41.
Nićiforović N., Abramovič H., 2014. Sinapic acid and its derivatives: natural sources and bioactivity. Compr. Rev. Food Sci. Foofd Saf. 13, 34–51, https://doi.org/10.1111/1541-4...
42.
NRC (National Research Council), 2012. Nutrient requirements of swine. 11th Revised Edition. National Academies Press, Washington, DC (USA), https://doi.org/10.17226/13298
43.
Onarman Umu Ö.C., Fauske A.K., Akesson C.P, Perez de Nanclares M., Sorby R., McLean Press C., Overland M., Sorum H., 2018. Gut microbiota profiling in Norwegian weaner pigs reveals potentially beneficial effects of a high-fiber rapeseed diet. PLOS ONE 13, e0209439, https://doi.org/10.1371/journa...
44.
Osman N., Adawi D., Molin G., Berggren A., Jeppsson B., 2006. Bifidobacterium infantis strains with and without a combination of oligofructose and inulin (OFI) attenuate inflammation in DSS-induced colitis in rats. BMC Gastroenterol. 6, 31, https://doi.org/10.1186/1471-2...
45.
Palade L.M., Habeanu M., Marin D.E., Chedea V.S., Pistol G.C., Grosu I.A., Gheorghe A., Ropota M., Taranu I., 2019. Effect of dietary hemp seed on oxidative status in sows during late gestation and lactation and their offspring. Animals 9, 194, https://doi.org/10.3390/ani904...
46.
Pérez de Nanclares M., Marcussen C., Tauson A.-H., Hansen J.Q., Kjos N.P., Mydland L.T., Bach Knaussen K.E., Overland M., 2019. Increasing levels of rapeseed expeller meal in diets for pigs: effects on protein and energy metabolism. Animal 13, 273–282, https://doi.org/10.1017/S17517...
47.
Pié S., Lalles J.P., Blazy F., Laffitte J., Seve B., Oswald I.P., 2004. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 134, 641–647, https://doi.org/10.1093/jn/134...
48.
Pistol G.C., Marin D.E., Rotar M.C., Ropota M., Taranu I., 2020. Bioactive compounds from dietary whole grape seed meal improved colonic inflammation via inhibition of MAPKs and NF-κB signaling in pigs with DSS induced colitis. J. Funct. Foods 66, 103708, https://doi.org/10.1016/j.jff....
49.
Plaipetch P., Yakupitiyage A., 2011. Use of yeast-fermented canola meal to replace fishmeal in the diet of Asian sea bass Lates Calcarifer (Bloch, 1790). J. Aquac. Res. Development 03, https://doi.org/10.4172/2155-9...
50.
Plaipetch P., Yakupitiyage A., 2013. Effect of replacing soybean meal with yeast-fermented canola meal on growth and nutrient retention of Nile tilapia, Oreochromis niloticus (Linnaeus 1758). Aquac. Res. 45, 1744–1753, https://doi.org/10.1111/are.12...
51.
Pourahmad J., Shaki F., Tanbakosazan F., Ghalandari R., Ettehadi H.A., Dahaghin E., 2011. Protective effects of fungal β-(1→3)-Dglucan against oxidative stress cytotoxicity induced by depleted uranium in isolated rat hepatocytes. Hum. Exp. Toxicol. 30, 173–181, https://doi.org/10.1177/096032...
52.
Salva S., Villena J., Alvarez S., 2010. Immunomodulatory activity of Lactobacillus rhamnosus strains isolated from goat milk: impact on intestinal and respiratory infections. Int. J. Food Microbiol. 141, 82–89, https://doi.org/10.1016/j.ijfo...
53.
Satessa G.D., Tamez-Hidalgo P., Hui Y., Cieplak T., Krych L., Kjaerulff S., Brunsgaard G., Nielsen D.S., Nielsen M.O., 2020a. Impact of dietary supplementation of lactic acid bacteria fermented rapeseed with or without macroalgae on performance and health of piglets following omission of medicinal zinc from weaner diets. Animals 10, 137, https://doi.org/10.3390/ani100...
54.
Satessa G.D., Tamez-Hidalgo P., Kjaerulff S., Vargas-Bello-Perez E., Dhakal R., Nielsen M.O., 2020b. Effects of increasing doses of lactobacillus pre-fermented rapeseed product with or without inclusion of macroalgae product on weaner piglet performance and intestinal development. Animals 10, 559, https://doi.org/10.3390/ani100...
55.
Saw C.L., Wu Q., Kong A.N., 2010. Anti-cancer and potential chemopreventive actions of ginseng by activating Nrf2 (NFE2L2) anti-oxidative stress/anti-inflammatory pathways. Chin. Med. 5, 37, https://doi.org/10.1186/1749-8...
56.
Sougioultzis S., Simeonidis S., Bhaskar K.R., Chen X., Anton P.M., Keates S., Pothoulakis C., Kelly C.P., 2006. Saccharomyces boulardii produces a soluble anti-inflammatory factor that inhibits NF-κB-mediated IL-8 gene expression. Biochem. Biophys. Res.. Commun. 343, 69–76, https://doi.org/10.1016/j.bbrc...
57.
Suda Y., Sasaki N., Kagawa K. et al., 2021. Immunobiotic feed developed with Lactobacillus delbrueckii subsp. delbrueckii TUA4408L and the soymilk by-product Okara improves health and growth performance in pigs. Microorganisms 9, 921, https://doi.org/10.3390/microo...
58.
Taranu I., Habeanu M., Gras M.A., Pistol G.C., Lefter N., Palade M., Ropota M., Chdea V.S., Marin D.E., 2018. Assessment of the effect of grape seed cake inclusion in the diet of healthy fattening-finishing pigs. J. Anim. Physiol. Anim. Nutr. 102, e30–e42, https://doi.org/10.1111/jpn.12...
59.
Taranu I., Marin D.E., Pistol G.C., Motiu M., Pelinescu D., 2015. Induction of pro-inflammatory gene expression by Escherichia coli and mycotoxin zearalenone contamination and protection by a Lactobacillus mixture in porcine IPEC-1 cells. Toxicon 97, 53–63, https://doi.org/10.1016/j.toxi...
60.
Untea A.E., Criste R.D., Vladescu L., 2012. Development and validation of a microwave digestion - FAAS procedure for Cu, Mn and Zn determination in liver. Rev. Chim. 63, 341–346
61.
Valko M., Morris H., Cronin T.D., 2005. Metals, toxicity and oxidative stress. Curr. Med. Chem. 12, 1161–1208, https://doi.org/10.2174/092986...
62.
Villena J., Aso H., Rutten V.P.M.G., Takahashi H., van Eden W., Kitazawa H., 2018. Immunobiotics for the bovine host: their interaction with intestinal epithelial cells and their effect on antiviral immunity. Front. Immunol. 9, 326, https://doi.org/10.3389/fimmu....
63.
Wang H., Khor T.O., Saw C.L.L., Lin W., Wu T., Huang Y. Kong A.T., 2010a. Role of Nrf2 in suppressing LPS-induced inflammation in mouse peritoneal macrophages by polyunsaturated fatty acids docosahexaenoic acid and eicosapentaenoic acid. Molecular Pharmaceutics 7, 2185–2193, https://doi.org/10.1021/mp1001...
64.
Wang R., Shaarani S.M., Godoy L.C., Melikoglu M., Vergara C.S., Koutinas A., Webb C., 2010b. Bioconversion of rapeseed meal for the production of a generic microbial feedstock. Enzyme Microb. Technol. 47, 77–83, https://doi.org/10.1016/j.enzm...
65.
Xiao K., Song Z.-H., Jiao F.-L., Ke Y.-L., Hu C.-H., 2014. Developmental changes of TGF-β1 and Smads signaling pathway in intestinal adaption of weaned pigs. PLOS ONE 9, e104589
66.
Xue Z., Yu W., Liu Z., Wu M., Kuo X., Wang J., 2009a. Preparation and antioxidative properties of a rapeseed (Brassica napus) protein hydrolysate and three peptide fractions. J. Agric. Food Chem. 57, 5287–5293, https://doi.org/10.1021/jf9008...
67.
Xue Z., Yu W., Wu M., Wang J., 2009b. In vivo antitumor and antioxidative effects of a rapeseed meal protein hydrolysate on an S180 tumor-bearing murine model. Biosci. Biotechnol. Biochem. 73, 2412–2415, https://doi.org/10.1271/bbb.90...
68.
Yamada E.A., Sgarbieri V.C., 2005. Yeast (Saccharomyces cerevisiae) protein concentrate: preparation, chemical composition, and nutritional and functional properties. J. Agric. Food Chem. 53, 3931–3936, https://doi.org/10.1021/jf0400...
69.
Yang H., Wu F., Long L., Li T., Liao P, Liu H., Yin Y., 2016. Effects of yeast products on the intestinal morphology, barrier function, cytokine expression, and antioxidant system of weaned piglets. J. Zhejiang Univ. Sci. B 17, 752–762, https://doi.org/10.1631/jzus.B...
70.
Yoshida Y., Umeno A., Akazawa Y., Shichiri M., Murotomi K., Horie M., 2015. Chemistry of lipid peroxidation products and their use as biomarkers in early detection of diseases. J. Oleo Sci. 64, 347–356, https://doi.org/10.5650/jos.es...
71.
Yuan L., Chang J., Yin Q., Lu M., Di Y., Wang P., Wang Z., Wang E., Lu F., 2017. Fermented soybean meal improves the growth performance, nutrient digestibility, and microbial flora in piglets. Anim. Nutr. 3, 19–24, https://doi.org/10.1016/j.anin...
72.
Zhou Y. Guan X., Zhu W., Liu Z., Yu H., Wang H., 2014. Capsaicin inhibits Porphyromonas gingivalis growth, biofilm formation, gingivomucosal inflammatory cytokine secretion, and in vitro osteoclastogenesis. EJCMID 33, 211–219, https://doi.org/10.1007/s10096...
73.
Zhu J., Mingxing G., Ruili Z., et al., 2017. Effects of soybean meal fermented by L. plantarum, B. subtilis and S. cerevisieae on growth, immune function and intestinal morphology in weaned piglets. Microb. Cell Factories 16, 191, https://doi.org/10.1186/s12934...
74.
Zou T., Yang J., Guo X., He Q., Wang Z., You J., 2021. Dietary seaweedderived polysaccharides improve growth performance of weaned pigs through maintaining intestinal barrier function and modulating gut microbial populations. J. Anim. Sci. Biotechnol. 12, 28, https://doi.org/10.1186/s40104...
CITATIONS (7):
1.
Yeast-Fermented Rapeseed Meal Extract Is Able to Reduce Inflammation and Oxidative Stress Caused by Escherichia coli Lipopolysaccharides and to Replace ZnO in Caco-2/HTX29 Co-Culture Cells
Ionelia Taranu, Gina Pistol, Andrei Anghel, Daniela Marin, Cristina Bulgaru
International Journal of Molecular Sciences
Ionelia Taranu, Gina Pistol, Andrei Anghel, Daniela Marin, Cristina Bulgaru
International Journal of Molecular Sciences
2.
Co-Contamination of Food and Feed with Mycotoxin and Bacteria and Possible Implications for Health
Daniela Marin, Gina Pistol, Cristina Procudin, Ionelia Taranu
Agriculture
Daniela Marin, Gina Pistol, Cristina Procudin, Ionelia Taranu
Agriculture
3.
Effects of a Curcumin/Silymarin/Yeast-Based Mycotoxin Detoxifier on Redox Status and Growth Performance of Weaned Piglets under Field Conditions
Vasileios G. Papatsiros, Georgios I. Papakonstantinou, Nikolaos Voulgarakis, Christos Eliopoulos, Christina Marouda, Eleftherios Meletis, Irene Valasi, Polychronis Kostoulas, Dimitrios Arapoglou, Insaf Riahi, Georgios Christodoulopoulos, Dimitra Psalla
Toxins
Vasileios G. Papatsiros, Georgios I. Papakonstantinou, Nikolaos Voulgarakis, Christos Eliopoulos, Christina Marouda, Eleftherios Meletis, Irene Valasi, Polychronis Kostoulas, Dimitrios Arapoglou, Insaf Riahi, Georgios Christodoulopoulos, Dimitra Psalla
Toxins
4.
ASSESSMENT OF CHEMICAL AND NUTRITIONAL QUALITY OF RAPESEED MEAL INTENDED FOR MONOGASTRIC LIVESTOCK FEEDING
Ionela Hotea, Catalin Sirbu, Anamaria Plotuna, Emil Tirziu, Isidora Radulov
23rd SGEM International Multidisciplinary Scientific GeoConference Proceedings 2023, Nano, Bio, Green and Space: Technologies for a Sustainable Future, Vol. 23, Issue 6.2
Ionela Hotea, Catalin Sirbu, Anamaria Plotuna, Emil Tirziu, Isidora Radulov
23rd SGEM International Multidisciplinary Scientific GeoConference Proceedings 2023, Nano, Bio, Green and Space: Technologies for a Sustainable Future, Vol. 23, Issue 6.2
5.
Effects of Low-Protein Diet Supplemented with Fermented Feed on Meat Quality, Fatty Acid Composition, and Gut Microbiota in Growing–Fattening Pigs
Qidong Zhu, Xiaorong Zhou, Dingbiao Long, Laifu Leng, Rong Xiao, Renli Qi, Jing Wang, Xiaoyu Qiu, Qi Wang
Agriculture
Qidong Zhu, Xiaorong Zhou, Dingbiao Long, Laifu Leng, Rong Xiao, Renli Qi, Jing Wang, Xiaoyu Qiu, Qi Wang
Agriculture
6.
Could fermentation of soybean and rapeseed meal be an avenue for innovation in French pig feed?
Valérie Heuzé, Patrick Carré, Isabelle de La Borde, Elodie Tormo, Gilles Tran
OCL
Valérie Heuzé, Patrick Carré, Isabelle de La Borde, Elodie Tormo, Gilles Tran
OCL
7.
Probiotics and oilseed cakes as feed additives in piglets nutrition
Mihaela Dumitru, Dan Râmbu, Georgeta Ciurescu
Archiva Zootechnica
Mihaela Dumitru, Dan Râmbu, Georgeta Ciurescu
Archiva Zootechnica
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.